How fast do Molecules dance?
Did you ever wonder how molecules interact with each other?
One thing about chemistry and physics is, that the underlying processes are rarely visible to the human eye. We talked about radioactive materials, which emit a deadly radiation. The downside is that we'll never see this radiation. Many reactions in chemistry are not visible. We don't see what the molecules do to each other. Especially when dealing with organic compounds we use a colorless liquid to create another colorless liquid. So today we will dive deep beyond what is visible for us to explore the nature of chemical reactions.
In chemistry there is a whole subtopic focused on reaction kinetics. Kinetics describe the way chemical reactions proceed - how fast they are, on what they are dependent etc... I'm trying to avoid those mathematical formulas which are normally used to describe those. In Kinetics we speak of reactions of first, second and third order - sometimes also zero order or fourth. You get the point. The order of a reaction describes the dependence of a reaction on the concentration of it's educt. What is an educt? Well in a chemical reaction we have some substances (educts, A & B) that create new ones (products, C). For example:
A + B --> C
Now a reaction of first order describes that the reaction speed is interdependent on the concentration of one educt. A good example therefore is radioactive decay.
²³⁸U --> ²³⁴Th + 𝛼
Uranium decays to Thorium while emitting 𝛼-particle. This reactions solely depends on the concentration of Uranium. So if I wanted to create 𝛼-particles fast, I would need to add more Uranium to the reaction.
Looking for a reaction of second order gets little more complicated. But when we turn to organic chemistry, there is a solution named nucleophile substitution which also occurs as a reaction of second order.
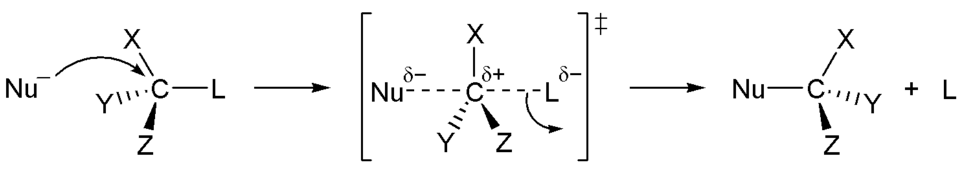
In this reaction the nucleophile attacks a carbon compound and forces the leaving group (L) to leave. This is a reaction of second order, where the speed is dependent on the concentration of the nucleophile (Nu) and the concentration of our carbon compound. A reaction of third order if we had three compounds that a relevant for our reaction mechanism.
Now we got the basics covered. We know how reaction orders work. Of course from her it only gets more complicated. There is also such a thing as pseudo orders, where technically we have a order of higher ranking, such as second, third or fourth, but one of our educts is present in a much higher concentration. If we take the example of our nucleophile substitution, we need a ration of 1:1 of our nucleophile and our carbon compound for the reaction to go as planned. If we had a ration of 10:1 or 100:1 the concentration of our nucleophile becomes irrelevant for our reaction, since we got so much of it, that now it only relies on the carbon compound. This would be a reaction of pseudo first order, since the concentration of our other educt stays mostly constant.
Now how do I determine reaction orders and the general kinetics of a reaction. Well I will give you an example, how I did it once in the lab. First of all we need a compound that has a dark color (I used crystal violet) which reacts with another molecule to a colorless compound. We will give them both in a solution and use a UV/Vis-Spectrometer to find out how much light is passing through our liquid. Thanks to Lambert-Beers-Law we are able to connect the loss of light to the concentration.
- E = Extinction
- ε = molar Coefficient of extinction (L·mol⁻¹·cm⁻¹)
- c = Concentration (mol/L)
- d = Thickness of liquid
- I₀ = Light entering our solution
- I = Light leaving the solution
Lambert-Beers-Law describes the passage of light through an absorbent solution. The extinction is proportional to the concentration of the liquid.
So when our extinction gets lower, we know our concentration of crystal violet gets smaller since its the only substance that absorbs light in our solution. This allows us to view the change of concentration over time. While crystal violet 'fades' in the presence of an alkaline, we can se how the reaction changes, when we change the concentration of that alkaline. Well turns out it changes a bit, but not much, because we are working with a strong dosage of alkaline and only a little bit of crystal violet. So we can work with a pseudo order, to identify that the reaction is dependent on the concentration of both educts. So the reaction over all is a reaction of second order.
Now it gets interesting because we can see how different other substances may influence the kinetics of our reaction. It becomes visible when you lay the extinction over time in one graph.
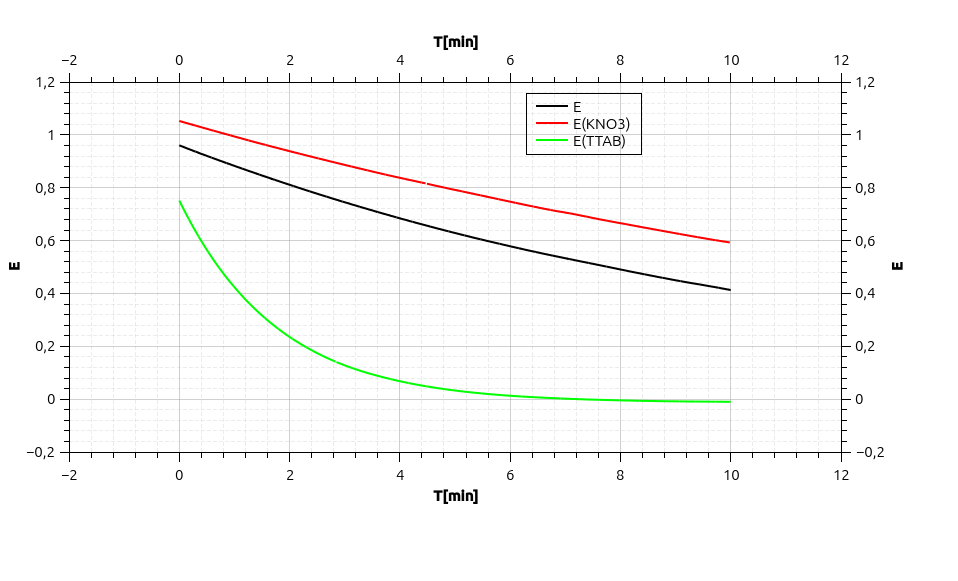
If we add another salt like potassiupotam nitrate (KNO₃) our reaction gets much slower. But if we add a tensid (or soap) like tetradecyltrimethylammoniumbromide (TTAB) it gets much faster. These are two well known effects on reaction kinetics.
First the kinetic salt effect describes, that a reaction is hindered in the presence of ions in the solution which are not involved in the reaction. This can be explained by the Debye-Hückel-Theory. Each Ion in solution is surrounded by a shell of hydrates and develops a cloud of ions around it; Crystal violet naturally is surrounded by a cloud of H₂O and OH⁻. We know that OH⁻ and crystal violet are going to react with each other, so its a good thing they surround each other. But now we got other ions in the solution which also develop ion clouds around the ions of the opposite charge. This is what inhibits our reaction, because KNO₃⁻ doesn't react with crystal violet and thus is only in the way of the interaction of crystal violet and OH⁻.
If we take a look at tetradecyltrimethylammoniumbromide (TTAB) and what is does to our reaction things get a little more interesting. Within a short amount of time our crystal violet is completely annihilated. This effect is called micellar catalysis. Tensides (containing a hydrophilic head and a hydrophobic tail) create micells in a watery solution. In this micells the hydrophobic tails look to each other and the heads are turned outward building little ball. Through this reactants can enrich at the boundary surface of this micells and thus enabling them to react faster. J. Albrizzio and colleagues investigated this specific reaction and found out, that the longer the tail of our tenside is, the faster the reaction runs.
So lets wrap this up. We have learned quite a bit about how molecules dance. In solution the boundary surface of them connect and the can react with each other. There are a lot of orders in kinetics to know about. And if we have a simple reaction we can do many great things to slow or speed up our reactions - if we know what we are doing. I hope you found this ride enjoyable, if so please let me know in the comments below.
See you soon, Nils

